|
Add This simple device to your lab water system
to save $1000's a year on replacement lab water filters |
What are the chemical properties of water
What are the physical and chemical properties of water that make it so unique and necessary for living things? When you look at water, taste and smell it - well, what could be more boring? Pure water is virtually colorless and has no taste or smell. But the hidden qualities of water make it a most interesting subject.
Also See :
Physical properties of domestic water
Water's Chemical Properties
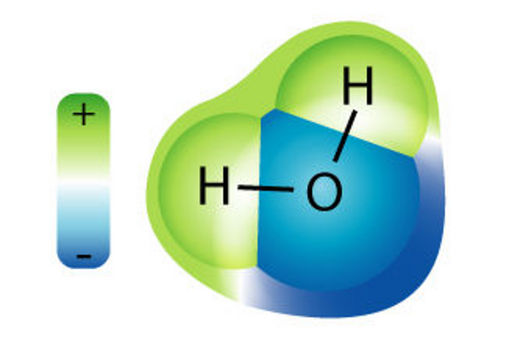 | You probably know water's chemical description is H2O. As the diagram to the left shows, that is one atom of oxygen bound to two atoms of hydrogen. The hydrogen atoms are "attached" to one side of the oxygen atom, resulting in a water molecule having a positive charge on the side where the hydrogen atoms are and a negative charge on the other side, where the oxygen atom is. Since opposite electrical charges attract, water molecules tend to attract each other, making water kind of "sticky." As the right-side diagram shows, the side with the hydrogen atoms (positive charge) attracts the oxygen side (negative charge) of a different water molecule. (If the water molecule here looks familiar, remember that everyone's favorite mouse is mostly water, too). |
All these water molecules attracting each other mean they tend to clump together. This is why water drops are, in fact, drops! If it wasn't for some of Earth's forces, such as gravity, a drop of water would be ball shaped -- a perfect sphere. Even if it doesn't form a perfect sphere on Earth, we should be happy water is sticky.
Water is called the "universal solvent" because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
Pure water has a neutral pH of 7, which is neither acidic nor basic.
Water's Physical Properties
Also See :
Physical properties of domestic water
Water is unique in that it is the only natural substance that is found in all three states -- liquid, solid (ice), and gas (steam) -- at the temperatures normally found on Earth. Earth's water is constantly interacting, changing, and in movement.
Water freezes at 32°Fahrenheit (F) and boils at 212°F (at sea level, but 186.4° at 14,000 feet). In fact, water's freezing and boiling points are the baseline with which temperature is measured: 0° on the Celsius scale is water's freezing point, and 100° is water's boiling point. Water is unusual in that the solid form, ice, is less dense than the liquid form, which is why ice floats.
Water has a high specific heat index. This means that water can absorb a lot of heat before it begins to get hot. This is why water is valuable to industries and in your car's radiator as a coolant. The high specific heat index of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans.
Water has a very high surface tension. In other words, water is sticky and elastic, and tends to clump together in drops rather than spread out in a thin film. Surface tension is responsible for capillary action, which allows water (and its dissolved substances) to move through the roots of plants and through the tiny blood vessels in our bodies.
Here's a quick rundown of some of water's properties:
Weight: 62.416 pounds per cubic foot at 32°F
Weight: 61.998 pounds per cubic foot at 100°F
Weight: 8.33 pounds/gallon, 0.036 pounds/cubic inch
Density: 1 gram per cubic centimeter (cc) at 39.2°F, 0.95865 gram per cc at 212°F
Here are some water volume comparisons:
1 gallon = 4 quarts = 8 pints = 128 fluid ounces = 231 cubic inches
1 liter = 0.2642 gallons = 1.0568 quart = 61.02 cubic inches
1 million gallons = 3.069 acre-feet = 133,685.64 cubic feet
ref:
http://ga.water.usgs.gov/edu/waterproperties.html
|

Images are representative of the products. Images may or may not be of the actual product. If it is important e-mail us for an actual image if available.
* Flat Rate UPS shipping when able to ship via UPS and is in the USA excluding Hawaii and Alaska.
Larger Items may not be able to ship via UPS, in that case freight charges will be quoted seperately.
International shipping will be quoted after the order is placed. You will have the opportunity to cancel before we finalize your order.
Terms and conditions
Credit Application
Privacy
Policy
Google Apps
List All Products
|