|
Need a New Laboratory Water System?
We have many to choose from. We can save you thousands on
Complete Systems and replacement filters for most brands. |
Molecular Structure Of Water
|
As atoms of two or more elements combine and join chemically, the product of the reaction is a new substance or compound, comprised of molecules. There are many different methods of combining atoms that result in the formation of molecules. Atoms either give up or acquire outer (or valence) electrons, this is known as ionic bonding. When atoms give up electrons they become positive ions also known as cations. Cations in a solution will be drawn to the cathode or negative pole of a battery. Conversely atoms that acquire electrons become negative ions also known as anions. Anions in a solution will be drawn to the anode or positive pole of a battery.
Chlorine and sodium (table salt)are held together by an ionic bond. The sodium atom gives up a single electron in its outer shell to chlorine, whose outer shell lacks one electron.
Another method by which atoms combine is known as the covalent bond. More common than ionic bonding, covalent bonding occurs when atoms share a pair of electrons. Hydrogen(H2)and Oxygen(O2)molecules are a result of covalent bonding. Water (H2O) is the result of the covalent between a single oxygen atom and two hydrogen atoms.
The water molecule formed through covalent bonding is known as a polar molecule. Each molecule has both positive and negative poles because the positive and negative changes are unevenly distributed. The equilibrium position of the hydrogen atom as it relates to the oxygen atom is a configuration that resembles the head of Mickey Mouse with Mickey's ears spearated at a 105o angle.
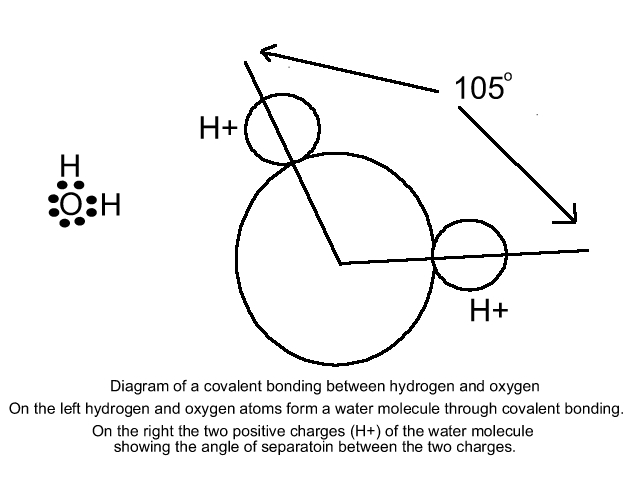
Due to this asymmetrical arrangement water molecules when in an electric field tend to orient themselves with the positively charged hydrogens towards the negative pole, and the negatively charges oxygen towards the positive pole. This is a common tendency in water and is known as the dipole moment, and the result is water having a large dielectric constant.
This dielectric constant makes water an excellent solvent, especially for compounds such as sodium chloride that are formed from ionic bonds. However these bonds are not strong thus in water the dissociated oppositely charged chloride and sodium ions remain dissociated due to waters strong dielectric constant. The bonds are essentially neutralized. The dissociated ions even tend to be attracted by the opposite charged ends of the water molecules in the solution.
It should be noted that capability of water as a solvent is not exclusive on its power to dissociate ionic bonds. It depends on another kind of the bond the hydrogen bond which joins water molecule to water molecule with remarkable strength.
Another weak intermolecular force is known as Van der Waals attraction. This is a result of from the attraction of one molecules nucleus for the electrons of another molecule and the attraction depends on the closeness of the molecules. The strength of this attraction must be overcome by thermal energy in order for evaporation to occur from the surface of the liquid. This attraction also determines the freezing point and boiling point of liquids, except for water.
As a general rule the larger the molecules nucleus the larger the van der Waal attraction of the molecules. With this generalization one can approximate freezing and boiling points. Water should be -100o C and -80oC yest based on measurement the temperatures are actually 0o and 100oC. Since this generalization does not hold for water another force must be at work here. The force at work is the hydrogen bond referred to above.
The unexpected freezing and boiling point of water is due to the hydrogen bonding. Hydrogen also helps to make water unusual. For instance it has a remarkable heat capacity and has high latent heats of fusion and evaporation. Waters ability to form hydrogen bonds is the because of its high surface tension. This also contributes to its adherence to other substances such as the walls of a container or fibers in fabric. In surface tension the hydrogen bonds form only between the water molecules. In adhesion the bonds form between the water molecules and the molecules of a second substance.
Hydrogen bonding also causes ice to float in water. Water contracts when it cools increasing its density. However at 4oC water will begin expanding thus decreasing in density. The tendency of water to contract as molecular motion decreases is overcome by the strength of the hydrogen bonding between the water molecules. At 4oC water molecules will begin to structure themselves directionally like hydrogen bonds at angles of 105o. When the temperature drops toward 0oC spaces develop between the lines until the open structure of the crystalinne characteristic of ice appears. Due to its openness the density is a little less than that of liquid water. Ice floats on the surface of water with approximately nine-tenths submerged.
More Information About Water
Molecular Structure
The Three States
Sublimation
Supercooled Water
Heavy Water
Properties
Heat Capacity
Latent Heats
Solvent
Surface Tension
|
|
|

Images are representative of the products. Images may or may not be of the actual product. If it is important e-mail us for an actual image if available.
* Flat Rate UPS shipping when able to ship via UPS and is in the USA excluding Hawaii and Alaska.
Larger Items may not be able to ship via UPS, in that case freight charges will be quoted seperately.
International shipping will be quoted after the order is placed. You will have the opportunity to cancel before we finalize your order.
Terms and conditions
Credit Application
Privacy
Policy
Google Apps
List All Products
|